Description
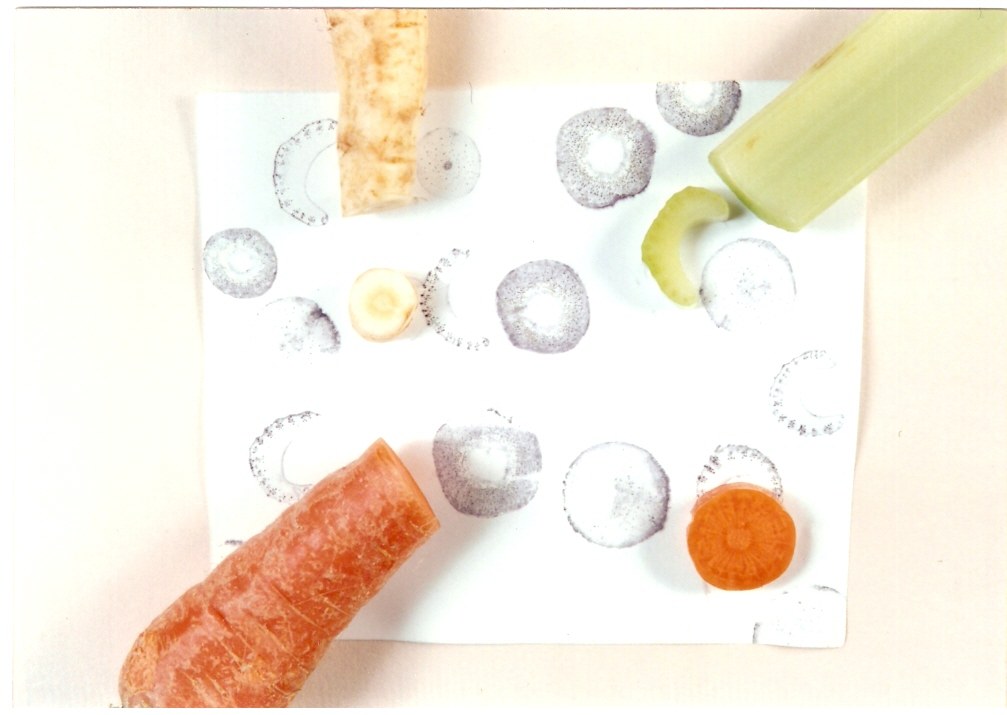
Locating specific proteins and nucleic acid molecules in tissue sections is an important goal in cell biology. An effective and simple technique for this purpose is tissue printing which permits the localization of specific macromolecules in animal and plant tissues. Here students perform this technique to examine the tissue distribution of the enzyme peroxidase in plants. First, students section carrots, celery, and other vegetables with razor blades and transfer the proteins from the cut sections to nitrocellulose membranes by application of gentle pressure. The enzyme peroxidase is detected on the membranes using a blue color producing peroxidase substrate as shown on the left. All proteins on the membranes are then stained red with a general protein stain which highlights the selective nature of the peroxidase distribution. Students also determine the amount of peroxidase in plant extracts. This 2 hour exercise provides an exciting lesson in plant histology and introduces your student to an important technique used in contemporary cell biology. The exercise was designed for eight groups of students and includes: nitrocellulose membranes, transfer pipets, 200 x extraction buffer, peroxidase standard, chloronapthol, 4M Tris buffer, hydrogen peroxide, and 10 x protein blot stain. Assorted vegetables which can be purchased at your local supermarket and petri dishes are needed, but not provided.
Sample
Here is a sample from the student manual:
IND-2. TISSUE PRINTING
Background Information
A. BASIC ANATOMY OF COMMON VEGETABLES
The edible portions of plants are usually divided into fruits and vegetables. Fruits are formed from a matured ovary or from an ovary and associated parts. Examples of fruits include apples, pears, tomatoes, and corn. Any edible part of a plant that is not a fruit is generally called a vegetable. Vegetables can be roots (e.g. carrots, parsnips), normal stems (e.g. asparagus), storage stems or tubers (e.g. potatoes), leaf stalks or petioles (e.g. celery), leaves (e.g. cabbage, lettuce), bulbs (e.g. onions), buds (e.g. brussel sprouts), and flowers (e.g. artichokes). Thus, a basic understanding of plant anatomy and taxonomy is needed for an understanding of the structure of vegetables.
Flowering plants can be subdivided into two groups, the monocotyledons and the dicotyledons. In monocotyledons, the embryo and seedling have one seed leaf or cotyledon. Plants in the lily, palm, grass, and orchid families are examples of monocotyledons. Two seed leaves are found in dicotyledons and this group includes carrots, potatoes, roses, oak trees, and most other herbs and woody plants. Monocotyledons and dicotyledons also differ in the structure of their stems, roots, and leaves and some of these differences are described below.
1. Development and Growth of Plants.
Different tissues of a plant arise by a process in which cell division is followed by cell growth and elongation and finally, by cell differentiation. The roots from seedlings or young bulbs have served as model systems for the study of these important processes. Several regions of the young root may be recognized by microscopy as shown in Figure 1. Cell division occurs near the tip (apex) of the root in a specialized tissue called meristem. The cells in this region, like those in the apical meristematic region of the stem, are in various stages of the cell cycle. The number of cells in the meristematic zone remains relatively constant, with one daughter remaining in the zone and the other entering the zone of elongation where cell elongation occurs. The elongation process involves true cell growth and is accompanied by large increases in cell dry weight and protein content. The zone of elongation merges into the zone of maturation, where the elongated cells begin to develop specialized structures and functions. This process, called cell differentia¬tion, leads to the formation of different tissues of the root including (1) the covering layer or epidermis which protects the soft interior of the root and which absorbs water and dissolved minerals from the soil, (2) the cortex which is chiefly a water and food storage region, and (3) the central vascular cylinder containing the xylem and phloem which serve to conduct water and nutrients through the plant. The xylem forms a continuous system of non-living water transport vessels. Water and inorganic ions ascend through these vessels in the root to the stern and into the leaves, from which the water is transpired (evaporated) into the air. The products of photosynthesis and other organic molecules are transported out of the leaves to other parts of the plant in the phloem.
In a typical case, a cell divides in the meristematic zone and one of the daughter cells elongates and then differentiates in the zones of elongation and maturation, respectively. The growth of the root and stem in length occurs chiefly by the elongation of cells that were produced in the apical meristems. This type of growth is referred to as primary growth and the different tissues that form are called the primary tissues. In most monocotyledons, cells in mature stems and roots are derived from the apical meristems and the tissues are therefore primary in origin. However, as dicotyledons grow older, they develop cambium in the root and stem. Cells within the cambium divide and differentiate producing secondary tissues which increase the diameter of the plant. Thus, in dicotyledons, most of the tissues of the mature root and stem are derived from the cambium rather than from the apical meristems.
B. DETECTION OF PEROXIDASE BY TISSUE PRINTING
The enzyme that you will study in today’s laboratory is called peroxidase. Peroxidase catalyzes the oxidation of phenolic compounds at the expense of hydrogen peroxide (11202). Although hundreds of papers have been published on peroxidase, the precise functions of the enzyme are uncertain. In plant systems, peroxidase is likely to play a role in synthesis of the plant cell wall. Here, the enzyme cross links phenolic residues of cell wall polysaccharides and glycoproteins which serves to strengthen the cell wall components. Peroxidase can also kill microorgan¬isms and destroy chemicals that are toxic to both plant and animal cells including 1I202 phenols, and alcohol. For these reasons, it has been proposed that peroxidase protects cells from microorganisms and toxic chemicals.
In this laboratory, you will localize peroxidase in vegetables by a tech-nique called tissue printing. This technique can be used to localize specific enzymes, antigens, and nucleic acid molecules in animal and plant tissues. You will section vegetables with a razor blade and transfer the proteins from the cut tissue sections to a nitrocellulose membrane by application of gentle pressure. An imprint of the tissue proteins will be formed on the nitrocellulose membrane. The enzyme peroxidase will then be detected on the membrane by the reaction shown in Figure 4. The nitrocellulose membrane will be incubated with chloronapthol and hydrogen peroxide. The peroxidase converts the chloronapthol to an insoluble purple product which is deposited at the site of the enzyme.
Objective
To determine the location of peroxidase in selected vegetables by tissue printing and to determine the amount of peroxidase in vegetable cell-free extracts.
Materials Provided
*Peroxidase Standards – The standards contain peroxidase isolated from horse radish. The concentration of peroxidase in the four standards is given below.
Standard Concentration of
Number Horse Radish Peroxidase
(gg peroxidase per ml)
1 0.01
2 0.1
3 1
4 10
*Extraction Buffer – The buffer (2mM MgC12, 20mM NaC1, 0.01%NP-40, 10mM Tris, pH 8.0) will be used to prepare the vegetable extracts.
*Color Development Solution – Prepared immediately before use by adding 5m1of chloronapthol, 0.7m1 of hydrogen peroxide, and 0.7m1 of 4M Tris buffer to 150m1 of distilled water.
*Protein “Blot” Stain – Ponceau S
Nitrocellulose (8 sheets)
Transfer Pipets (10)
*Prepared as described in the Instruction Guide.
Materials Needed But Not Provided
For Tissue Printing:
Distilled or deionized water
Forceps or gloves for handling the nitrocellulose
Petri dishes
Razor blades
Metric rulers (8)
Paper towels
Hand lens (optional)
Assorted vegetables – Carrots, parsnips, asparagus, and celery are among
suitable vegetables for the exercise.
For Preparation and Analysis of Peroxidase in Vegetable Extracts:
One gram pieces of selected vegetables – These may be prepared prior to the
laboratory session.
Balance
Scissors (8)
Mortars and pestles (8)
Table-top centrifuge and centrifuge tubes (8)
Microliter dispensers (8)
Small (~1.5m1) tubes (16)
Procedure
I. Preparation of the Cell-Free Extract
1. Place one gram of a selected vegetable into a mortar and add 2m1 of enzyme extraction buffer.
2. Cut the tissue into smaller pieces with scissors.
3. Grind the tissue with the pestle until a homogenous suspension is formed. The mechanical action of the pestle and the chemical action of the detergent Nonidet P-40 that is present in the extraction buffer should disrupt the plant cells which in turn will liberate their cytoplasmic proteins.
4. Transfer the solution to a centrifuge tube, centrifuge it for 5 minutes, and remove the supernatant fraction (the top liquid) with a pipet. Place this solution into a small clean tube and label the tube “Vegetable Extract -100%”.
5. Transfer 5µl of this extract to a tube containing 45µ1 of distilled water. Label this tube “Vegetable Extract – 10%”.
II. Preparation of the Tissue Prints
1. Wet one sheet of nitrocellulose by floating it in a petri dish with about 20m1 of distilled water.
2. Place one moist paper towel flat on the laboratory bench in front of you. Place the nitrocellulose membrane on the paper towel.
Note: Gloves should be worn when handling nitrocellulose to prevent transfer of proteins from your hands to the membrane. If gloves are not available, use forceps. Touch only the edges of the membranes with gloves or forceps.
3. Remove excess moisture from the membrane by blotting with a dry paper towel.
4. Place a metric ruler next to the nitrocellulose membrane along one short edge as indicated in Figure 5.
5. Pipet 5µl of each of the four peroxidase standards (#1-4) onto the nitro¬cellulose about 1/2cm from the ruler. The standards should be carefully pipetted onto the membranes to form individual spots at 1cm, 2cm, 3cm, and 4cm along the edge of the membrane as indicated in Figure 5.
6. Rinse the pipet with water and then pipet 5p1 of “Vegetable Extract 10%” and 5p1 of “Vegetable Extract 100%” onto the membrane to form individ¬ual spots at 1cm from the edge of the membrane as indicated in Figure 5.
7. Allow about 5 minutes for the solutions to be absorbed onto the mem¬branes.
8. Chose up to four vegetables to be examined by tissue printing. These can either be four different smaller vegetables or 4 different sections along a single vegetable. Four different sections along a single carrot from tip to base provide an example of the latter.
8
9. Using a razor blade, cut the vegetable to produce a cross-section. Gently blot the cut surface onto a dry paper towel to remove excess liquid.
10. Position the cut surface of the vegetable onto the nitrocellulose and press down firmly for 10 seconds making sure not to move your hand during the process. Note: The cut surface should be applied at one of the 4 positions of the membrane indicated in Figure 5.
11. Remove the vegetable section and then repeat the process with 3 different vegetables or 3 different sections from the same vegetable. Note: Each tissue print must be placed on an unoccupied section of the nitrocellulose membrane as indicated in Figure 5.
12. After all tissue prints have been prepared, place the nitrocellulose mem¬brane in a petri dish with distilled water.
III. Detection of Peroxidase
1. Examine sections of plants that you prepared for tissue printing. With the aid of Figures 2 and 3 and a hand lens, (if available), attempt to identify epidermis, cortex, xylem, and phloem.
2. Examine the tissue prints to determine if imprints of these tissues can be seen on the nitrocellulose membranes.
3. Discard the water in the petri dish and place about 15m1 of freshly prepared color development solution into the dish.
4. Observe development of blue color (peroxidase activity) over the next 5 minutes.
5. After about 5 minutes discard the color development solution and add water to the petri dish.
6. In the space provided on the following page, draw diagrams of the tissue prints. On the diagrams, identify the tissues that contain peroxidase.
Peroxidase Standard
#1 (0.01µ/ml)
#2 (0.1µg/m1)
#3 (1µg/m1)
#4 (10µg/ml)
Vegetable Extracts 10% Extract 100% Extract
9 10
IV. Detection of Total Proteins
1. After discarding the water from the petri dish, add 15m1 of protein blot stain. This solution (Ponceau S) should stain all proteins on the nitrocel¬lulose membrane red.
2. After 5 minutes, pour off and discard the stain, wash the membrane 3 times with water and note the regions on the membrane that are red. Attempt to identify those regions on the membrane that stained red but were not stained with the color development solution.
3. The blot may be stored protected from heat and light (between two sheets of black construction paper, for example).
Study Questions
1. Name the tissues that contained the highest concentration of peroxidase.
2. Describe one function that peroxidase may perform in each of these tissues.
3. Name one plant tissue area that contained proteins that were transferred to the nitrocellulose but did not contain peroxidase.
4. A comparison of the relative intensities of blue spots produced by the peroxi-dase standards to the intensities of blue spots produced by the vegetable extracts should enable you to estimate the amount of peroxidase in the vegetable extracts.
A. Estimate the amount of peroxidase in lml of your undiluted vegetable extract.
B. Estimate the amount of peroxidase in one gram of the vegetable that was used as starting material for preparation of the extract.
 Due to Customs restrictions, we only accept orders from educational institutions within the Continental United States, Alaska or Hawaii.
Due to Customs restrictions, we only accept orders from educational institutions within the Continental United States, Alaska or Hawaii.