What Is A Peptide Bond Simple Definition?
What Is A Peptide Bond Simple Definition?
A very basic explanation could run something like this:
We tend to think of protein as a macronutrient, a kind of homogeneous substance that everyone needs in their diets. But as you have been learning in your modern biology labs, protein isn’t a single substance. There are lots and lots of proteins in different organisms, or in a single organism, or even in a single cell.
Proteins are the most abundant and the most diverse macromolecules in living organisms. They are all similar in that they are made of chains of amino acids, but they differ in the ways their constituent amino acids are connected by peptide bonds.
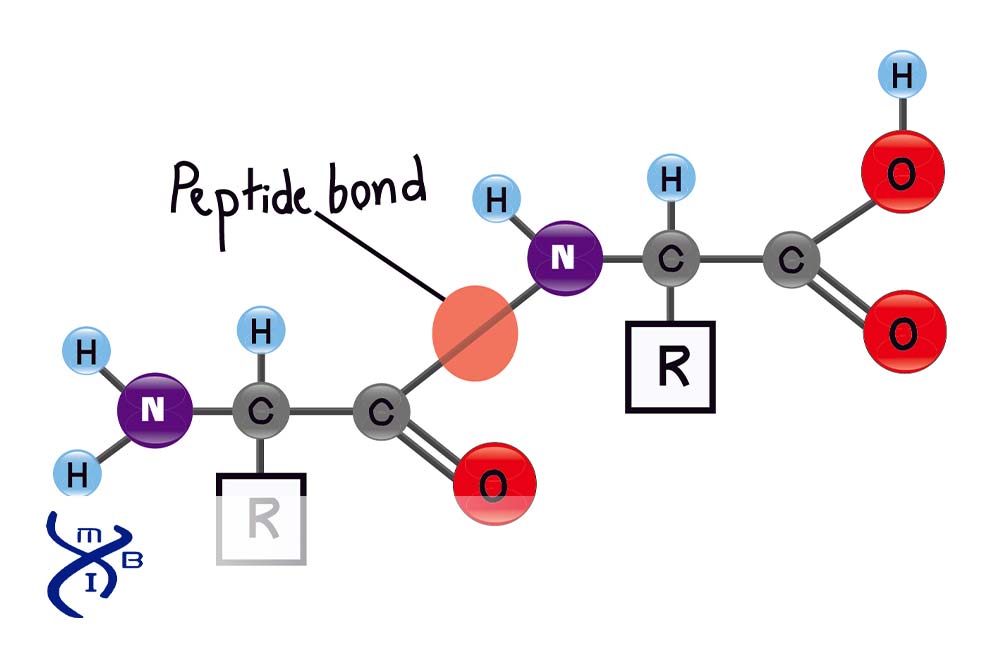
What Is a Peptide Bond?
Proteins consist of a specific order of amino acids joined by covalent peptide bonds.
Every cell uses tRNA to determine the sequence of amino acids to make proteins. These amino acids join the protein in order and are connected by a condensation (dehydration synthesis) reaction.
During protein synthesis, the -COOH (carboxyl) group of one amino acid reacts with the NH2 (amino) group of the next amino acid in the sequence. The carboxyl and amino groups link by releasing a molecule of water, H2O. The resulting chemical combination is known as a peptide, which may consist of two to thousands of amino acids joined by peptide bonds.
The precise definition of a peptide is a chain of amino acids. A peptide chain doesn’t really become a “protein” until it folds on itself to form a specific function in a living cell.
How Do You Identify a Peptide?
Identifying the peptide bond that make up a protein tells us more about the protein’s activity. It can reveal something about the genetic coding that was used to create it. Biologists can sever peptide bonds with heat and acid to break them back down into their constituent amino acids, but how do you study the sequence of amino acids in an intact protein chain?
Think about how a peptide is formed.
The very first amino acid in a peptide chain will lose its -COOH (carboxyl) group to form a covalent bond with the second amino acid in the chain, but its -NH2 (amino) group will be intact. This amino acid becomes known as the N-terminus of the peptide chain.
The very last amino acid in a peptide chain will lose its -NH2 (amino) group to form a covalent bond with the next-to-last amino acid in the chain before it, but its -COOH (carboxyl) group will remain intact. This amino acid becomes known as the C-terminus of the peptide chain.
How Do You Identify Peptide Bonds?
Identifying the terminal acids of a peptide chain, along with electrophoresis to measure the size of the peptide molecule, tells investigators a great deal about the function of the peptide in a protein.
What Are Some Examples of Peptide Bonds?
For instance, consider the tripeptide (three amino acid) molecule glutathione, which acts as an antioxidant and stimulates cell growth. This peptide consists of three amino acids, glutamic acid, cysteine, and glycine, with a chemical formula of (+)H3NCH(CO2(–))CH2CH2CONHCH(CH2SH)CONHCH2CO2H. Reactions with the N-terminus that identify glutamic acid and reactions with the C-terminus that identify glycine, plus some knowledge of the size of the molecule, assist in identification.
Students will discover that characterizing peptides is something they can do in the lab with Modern Biology’s Experiment 204, Peptide Mapping Analysis and Modern Biology’s Cell Biology and packages.
There has never been a more exciting time to teach modern biology. Join the 80,000 biology teachers nationwide who use Modern Biology products to train over 500,000 students in scientific method and essential lab technique. Email us or call us weekdays between 9 and 5 Eastern at (765) 446-4220 for more information or to place your order.
 Due to Customs restrictions, we only accept orders from educational institutions within the Continental United States, Alaska or Hawaii.
Due to Customs restrictions, we only accept orders from educational institutions within the Continental United States, Alaska or Hawaii.