Description
Objective: To study the effects of DNAse I and denaturation on the structure of DNA.
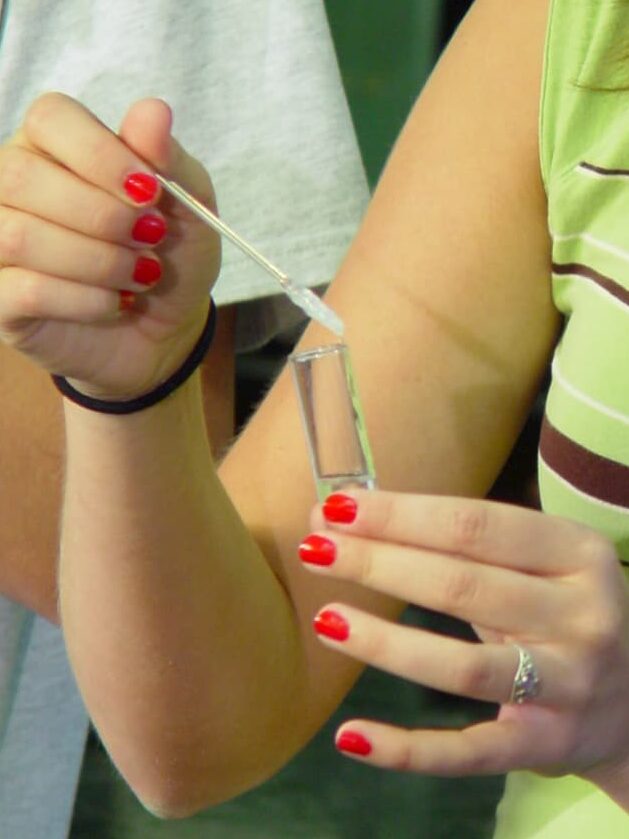
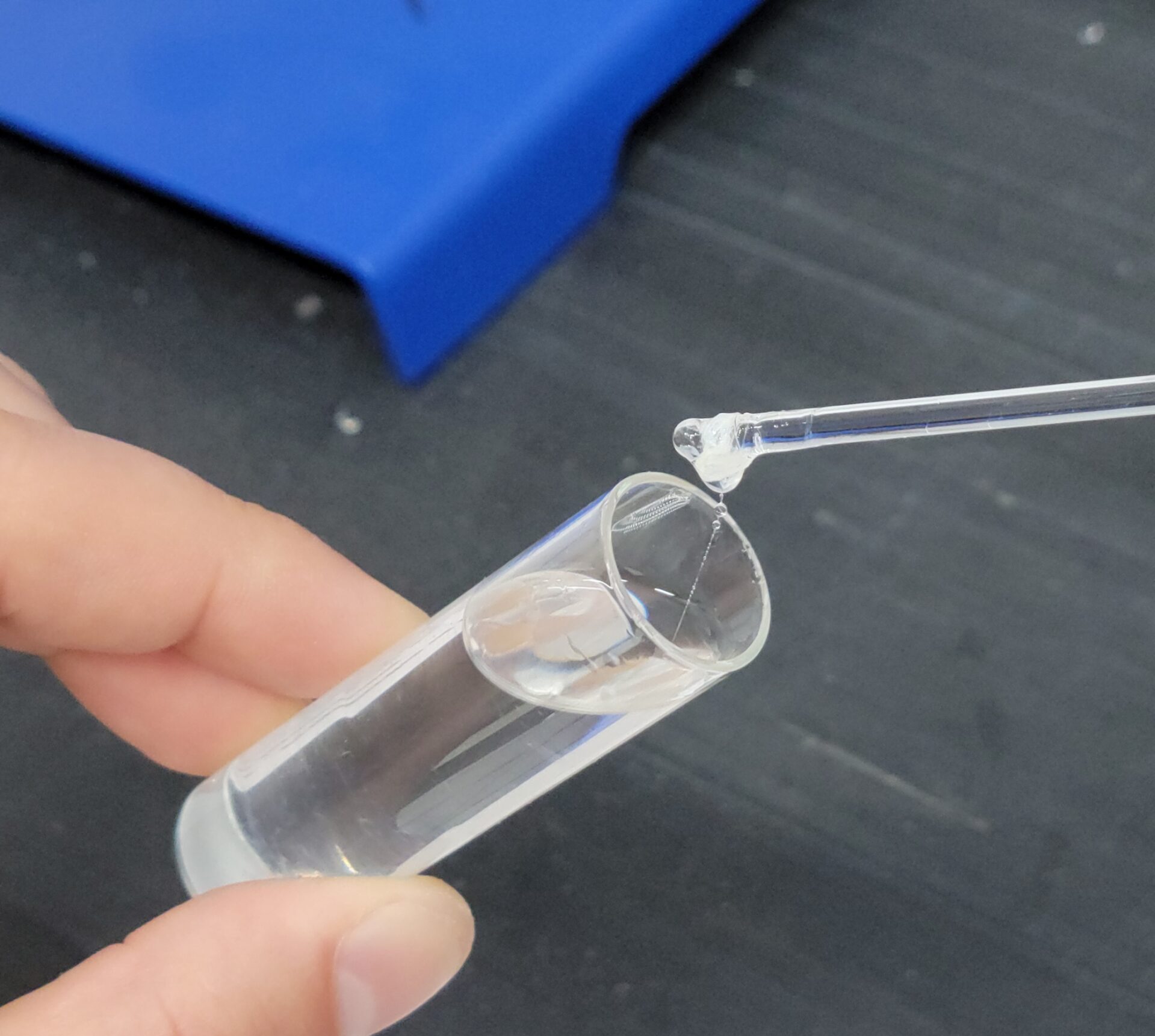
The DNA molecule from a single human chromosome is about 4 cm long and the length of DNA in an individual is about 200 times the distance from the earth to the sun. Isolated DNA in a test tube is also a long, stiff molecule. When alcohol is added to a DNA solution, the DNA fibers precipitate and can be spooled onto a glass rod. This feature of DNA is illustrated in the exercise, which provides enough purified DNA for 16 students working in pairs to perform the experiment. The spooling phenomenon is also used by the student to study the double-stranded nature of DNA and to investigate the effect of breaking DNA into small pieces with the enzyme DNAse.
Materials Provided
80 ml Calf Thymus DNA
1 ml DNAse I
8 glass vials
8 glass rods for DNA spooling
10 small transfer pipet – These pipets should be used for the DNAse I solution.
10 large transfer pipets
Materials Not Provided
Denatured alcohol
Ice chips in a beaker
Boiling water bath (A 250m1 beaker with water over a burner can be used.)
16 glass test tubes and a few metal tube holders
1% NaCI – (OPTIONAL – see below and Instructor Manual)
Sample
Experiment (B1-1). Properties of DNA
Background Information
DNA is an extremely long molecule that is very thin, yet quite rigid. The isolation of intact DNA molecules from a cell is difficult because of the relative ease with which these long rod-like molecules can be broken. Even the injection of a solution of DNA through the needle of a hypodermic syringe can cause extensive breakdown of DNA molecules. DNA can also be broken down by enzymes called deoxyribonucleases. Deoxyribonuclease I (DNAse I), an enzyme isolated from the mammalian pancreas, will be used in today’s experiment. This enzyme breaks the phosphodiester bonds that connect the nucleotide units in DNA, degrading long DNA molecules to a mixture of small nucleotide chains, as illustrated in Figure 1-1.
A DNA molecule is composed of two polynucleotide chains that are coiled around each other to form a rigid double-helix (see page 4). The double-helical structure of DNA is very stable at room temperatures because the hydrogen and hydrophobic bonds between the stacked bases hold the two polynucleotide chains together. However, if a solution of DNA is heated to a critical temperature, these bonds are broken and the two polynucleotide strands separate by a process called denaturation. DNA denaturation is accompanied by a decrease in the viscosity (thickness) of the solution because single-stranded DNA molecules form flexible coiled structures that no longer retain the rigid native structure of the DNA double-helix (Figure 1-2). If the DNA is cooled rapidly, the molecules will remain as single stranded polynucleotides. However, if the solution is cooled very slowly, restora¬tion of the DNA helix will occur. The reassembly of the two separated polynucleo¬tide strands is called renaturation.
Macromolecules such as DNA, RNA, and protein are not soluble in alcohol solutions, and precipitate (come out of solution) upon addition of alcohol. In general, globular proteins and RNA form fine, non-fibrous precipitates in alcohol. In contrast, the rod-like DNA molecules precipitate in alcohol as long fibers that can be spooled onto a glass rod. The ability of DNA to form fibers in alcohol depends on the physical properties of the DNA molecules. For example, DNA that has been broken into small pieces by DNAse I digestion will not form fibers, nor will single-stranded DNA that has been prepared by heat denaturation of the DNA double-helix. These properties of DNA will be illustrated in today’s experiments.
Study Questions and Analysis
1. Describe the basic properties of DNA that are responsible for fiber formation when alcohol is added to a solution that contains native (double-stranded) DNA.
2. Describe the type of precipitate that is formed when alcohol is added to denatured (single stranded) DNA. Offer an explanation as to why this precipi¬tate is different from that which is observed with native DNA.
3. Describe the action of DNAse Ion DNA and relate this effect to the results of your experiment in Section HI.
 Due to Customs restrictions, we only accept orders from educational institutions within the Continental United States, Alaska or Hawaii.
Due to Customs restrictions, we only accept orders from educational institutions within the Continental United States, Alaska or Hawaii.